Flame Tests & Bright-Line Spectra. Visible light is composed of
Flame Tests & Bright-Line Spectra It was Isaac Newton who proved that visible (“white”) light was composed of the basic colors red, orange, yellow, green, blue, and violet.
Flame Tests & Bright-Line Spectra
Visible light is composed of the basic colors red, orange, yellow, green, blue, and violet (R.O.Y.G.B.V.).
Flame Tests & Bright-Line Spectra It was Isaac Newton who proved that visible ( white ) light was composed of the basic colors red, orange, yellow, green, blue, and violet.
Flame Tests & Bright-Line Spectra Light is not just the visible light spectrum (R.O.Y.G.B.V.) but includes radiation such as TV and radio, microwaves, radar, infrared light, ultraviolet light, X-rays, and gamma rays.
10 –16 10 –14 10 –12 10 –10 10 –8 10 –6 10 –4 10 – wavelength (meters) frequency (hertz – cycles/s ) gamma rays X rays ultraviolet visible light infrared radar microwaves TV & radio waves 400 nm450 nm500 nm550 nm600 nm650 nm700 nm 750 nm redvioletbluegreenyelloworange wavelength (nm) Low EnergyHigh Energy.
Every photon has a specific frequency, wavelength, and energy. 10 –16 10 –14 10 –12 10 –10 10 –8 10 –6 10 –4 10 – wavelength (meters) frequency (hertz – cycles/s ) gamma rays X rays ultraviolet visible light infrared radar microwaves TV & radio waves 400 nm450 nm500 nm550 nm600 nm650 nm700 nm 750 nm redvioletbluegreenyelloworange wavelength (nm) Low EnergyHigh Energy.
Violet light is high energy visible light while red light is low energy visible light. Low EnergyHigh Energy 10 –16 10 –14 10 –12 10 –10 10 –8 10 –6 10 –4 10 – wavelength (meters) frequency (hertz – cycles/s ) gamma rays X rays ultraviolet visible light infrared radar microwaves TV & radio waves 400 nm450 nm500 nm550 nm600 nm650 nm700 nm 750 nm redvioletbluegreenyelloworange wavelength (nm) High EnergyLow Energy.
Barium PotassiumLithiumCopper Calcium StrontiumSodium.
Flame Tests & Bright-Line Spectra Road flares are a practical use of flame test colors.
Flame Tests & Bright-Line Spectra Fireworks are a spectacular example of flame test colors.
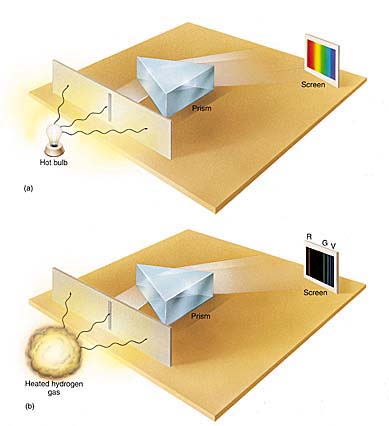
A spectroscope is an instrument that will separate light into its various wavelengths – thus into its various colors..
This is what scientists saw when looking at sunlight through a spectroscope:.
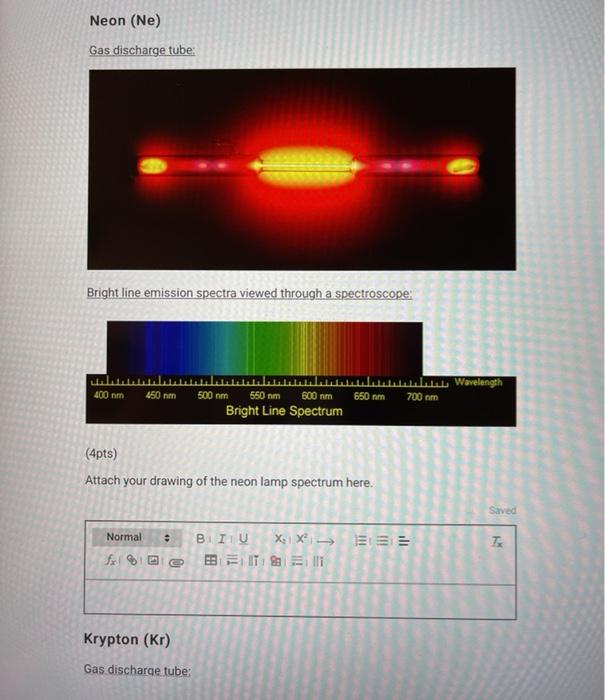
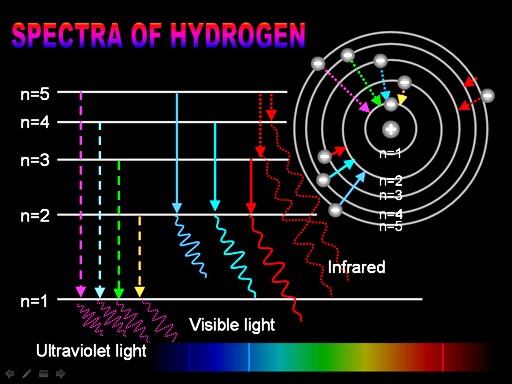
Flame Tests & Bright-Line Spectra This is what scientists saw in the spectro- scope when looking at glowing hydrogen gas: This is what scientists saw in the spectro- scope when looking at glowing sodium vapor: 400 nm500 nm600 nm700 nm sodium 400 nm500 nm600 nm700 nm hydrogen
Flame Tests & Bright-Line Spectra This is what scientists saw in the spectro- scope when looking at glowing mercury vapor: This is what scientists saw in the spectro- scope when looking at glowing lithium vapor: 400 nm500 nm600 nm700 nm mercury 400 nm500 nm600 nm700 nm lithium
Flame Tests & Bright-Line Spectra This is what scientists saw in the spectro- scope when looking at glowing helium gas: This is what scientists saw in the spectro- scope when looking at glowing cadmium vapor: 400 nm500 nm600 nm700 nm helium 400 nm500 nm600 nm700 nm cadmium
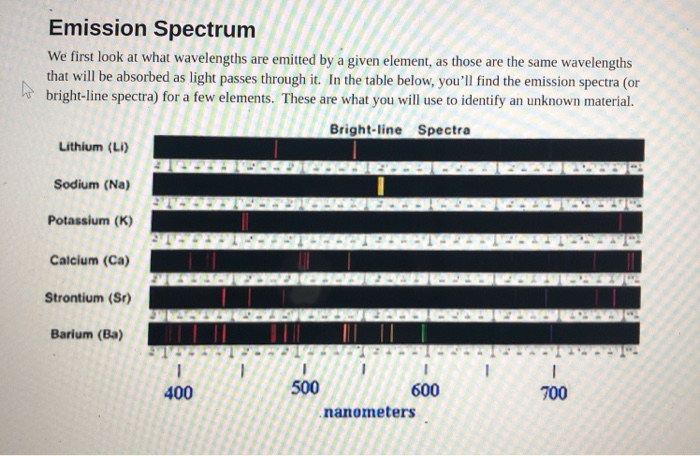
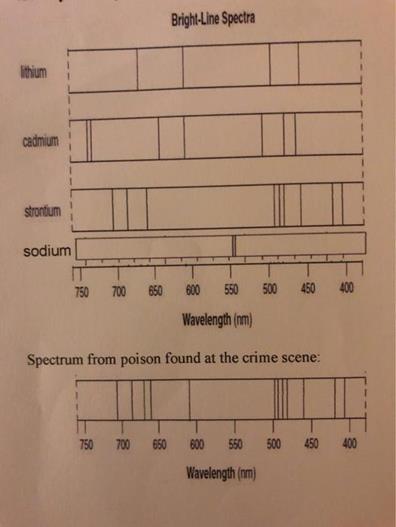
An element’s bright-line spectrum is like a fingerprint In that the pattern of lines at specific wavelengths can be used to identify the presence of an element..
A new element had been discovered. – helium.
At first, its was believed that helium was only found in the sun..
Helium is much less dense than air and so it is used in party balloons and in blimps..
For years it puzzled scientists why this was so. Since each element is composed of unique atoms It must be the atoms. How do the atoms of each element produce the element’s unique bright-line spectrum .

PPT - Flame Tests & Bright-Line Spectra PowerPoint Presentation - ID:2762632

flame-tests-and-atomic-spectra-labdoc.doc - Atomic Theory Part One: Flame Tests Part Two: Atomic Spectra This activity will focus on the visible portion
Chapter 4, Section 1
What is visible light, and how much of the spectrum does it cover compared to the others? - Quora

Emission spectrum - Wikipedia

flame-tests-and-atomic-spectra-labdoc.doc - Atomic Theory Part One: Flame Tests Part Two: Atomic Spectra This activity will focus on the visible portion

Visible Light
What is the difference between a line spectrum and a continuous spectrum? - Quora
Atomic spectra
Light and the Modern Atom

Flame Tests & Bright-Line Spectra. Visible light is composed of the basic colors red, orange, yellow, green, blue, and violet (R.O.Y.G.B.V.). - ppt download

Flame Tests & Bright-Line Spectra. Visible light is composed of the basic colors red, orange, yellow, green, blue, and violet (R.O.Y.G.B.V.). - ppt download